Adherence to international guidelines and best practices.
A track record of delivering impactful results for clients.

A dedicated team with extensive industry and site experience.
Services tailored to unique individual and organizational needs.
Our Organization is a trusted leader in delivering comprehensive educational and consultancy services to the clinical trial industry, backed by over two decades of expertise.
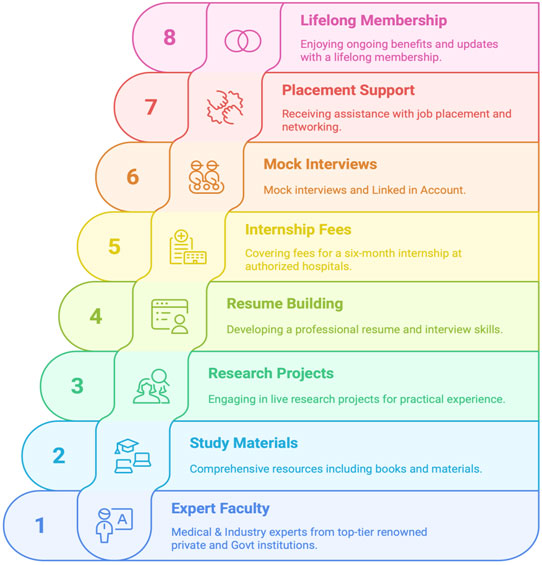
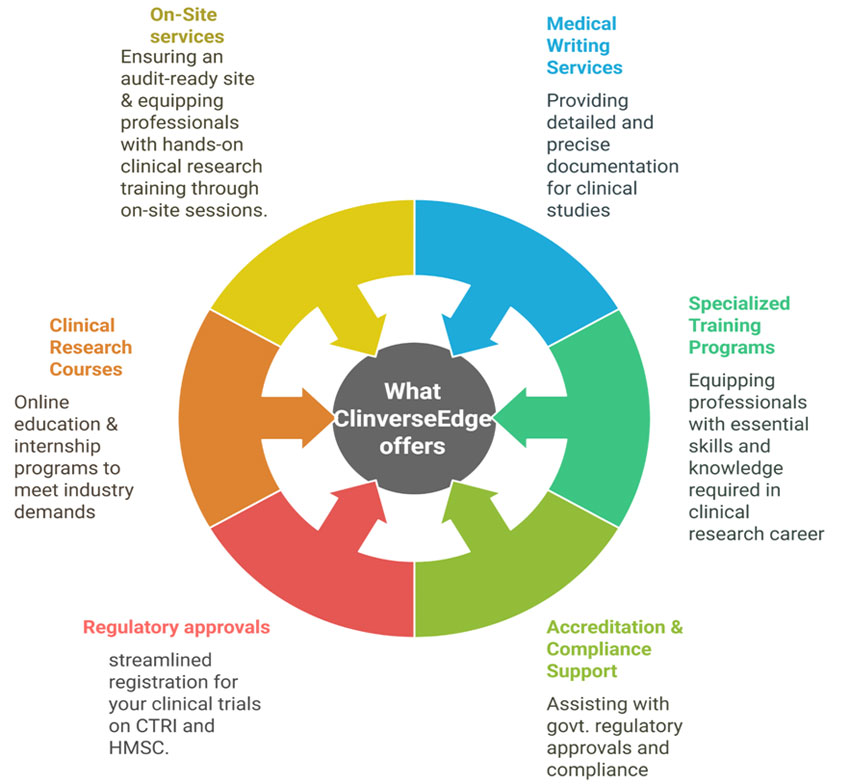
We specialize in education, medical and regulatory writing, SOP development, manuscript writing and on-site training.
Our training modules encompass ICH GCP, NDCT, ICMR guidelines, and advanced diploma in Clinical Research field. We provide expert support for regulatory approvals like CTRI and HMSC, site readiness for audits.
Read More

Countries (workshops)
CRO/Sponsor Audits
Trained Professionals
Original
Articles
Trained EC Members
International Trials Conduct
Clinical Trial site monitoring
Hear firsthand from clinical research site and industry experts as they share their experiences with our faculty and how our faculty played a pivotal role in mentoring them throughout their clinical research journeys.

Lead Oncology Researcher at the Sir H.N. Reliance Foundation Hospital and Research Center, Mumbai

BDS, Master in Clinical Research

MBBS, Clinical Researcher, Medical Writer Expert

BDS , Master in Clinical Research

B.Sc. MBA (Nanyang Technological University, Singapore)

Senior Executive- Clinical Research Dept Kokilaben Dhirubhai Ambani Hospital & Research Centre , Mumbai

Master in Pharmacy Senior Clinical Research Associate in Diagno Search Life Sciences , Mumbai

Senior Clinical Research Coordinator K J Somaiya Hospital & Research Centre Post graduation in clinical research

Master in Clinical Research, Mumbai
Note: Enroll by July 15th to get discounts on our clinical research courses—as we sincerely aim to support talented students in pursuing their ambitions affordably *fees includes GST