
Are you a science, pharma, or medical graduate wondering what’s next after your degree? Or maybe you’re a working professional looking for a better career that combines innovation, purpose, and global impact?
If yes, then a career in clinical research could be your perfect choice. It’s a field where you get to work on testing new medicines, vaccines, and medical devices for safety and effectiveness in humans.
The clinical research industry is booming worldwide, especially in India, where it is expected to grow by more than 12% annually. That means more job opportunities, better salaries, and a chance to be part of groundbreaking medical advancements. Whether you want to work in hospitals, research organizations, pharmaceutical companies, or government agencies, clinical research offers a wide range of roles suited to different interests and skills.
Clinical research is essential for developing new medicines and improving healthcare. It involves designing and conducting studies called clinical trials to test new treatments. Professionals working in this industry play a crucial role in advancing medical knowledge and helping patients worldwide.
So, what exactly are the career options in this exciting field? Let’s explore!
The field of clinical research offers a wide variety of roles, each with its own responsibilities and skills. No matter your background or interests, there is likely a role that suits you and helps you contribute to the advancement of medical science. Let’s explore some of the most common and important career options in this industry.
A Clinical Research Coordinator acts as a vital link between doctors, sponsors, and patients involved in a trial. They manage many day-to-day activities, such as recruiting participants, collecting data, and maintaining detailed records of the trial process. CRCs also ensure that the study follows ethical guidelines and complies with legal regulations. Their role is crucial in making sure the trial runs smoothly, ethically, and efficiently, all while maintaining good communication among everyone involved.
The Clinical Research Associate is responsible for overseeing the actual conduct of clinical trials at different research sites. They visit these sites regularly to verify that the trial is being conducted according to the protocol and regulatory requirements. CRAs check data accuracy, ensure the safety of participants, and confirm that all activities meet Good Clinical Practice (GCP) standards. They serve as a bridge between the trial sites and the sponsors, making sure everything runs smoothly and that any issues are quickly addressed.
The Clinical Trial Assistant provides essential administrative support for clinical trials. Their tasks include filing and organizing documents, preparing trial-related paperwork, scheduling meetings, and coordinating logistics. They help keep the trial organized by managing schedules, maintaining records, and assisting the team with day-to-day operational tasks. Their work ensures that all administrative aspects of the trial are handled efficiently, supporting the overall success of the study.
One key role in clinical research is the Clinical Data Coordinator. This professional is responsible for ensuring that all trial data is accurate, complete, and stored properly. They organize and maintain large databases, making sure that the information collected from various trial sites is consistent and reliable. Working closely with research teams, they collect, process, and verify data to meet strict regulatory standards. Their work guarantees that the information used to evaluate new medicines and treatments is trustworthy, which is vital for the success of clinical trials.
A Clinical Trials Manager takes charge of the entire trial process. They plan, organize, and monitor each step of the clinical trial, from beginning to end. This includes managing budgets, timelines, and resources, and making sure that the study complies with all legal and regulatory standards. The manager’s leadership ensures that the trial progresses smoothly, stays within budget, and produces reliable results.
Medical Writersensures that complex scientific data is communicated clearly, accurately, and in compliance with regulatory standards. They prepare detailed reports, research papers, regulatory submissions, and patient education materials. They take complex clinical data and interpret it into clear, understandable language. Their work helps communicate scientific results to regulators, healthcare professionals, and the public, ensuring that research findings are shared effectively.
The Project Manager in clinical research leads the planning, execution, and completion of trials. They coordinate teams, manage resources, and oversee timelines to ensure the study’s success. They handle communication among different departments and stakeholders, making sure that everyone works together toward a common goal. Their leadership is essential for delivering quality results on time and within budget.
Regulatory Affairs Specialists ensure that all aspects of a clinical trial are compliant with government and industry regulations. They prepare and submit necessary documents to regulatory authorities to gain approval for trials and new drugs. They also stay updated on changing regulations and advise the team on compliance issues, helping to avoid delays or legal problems.
These varied roles in clinical research open up many exciting career opportunities for students and professionals from different backgrounds. Whether you enjoy managing data, coordinating teams, writing reports, or analyzing results, there’s a place for you in this industry. With the right training and dedication, you can build a rewarding career that not only offers good salary prospects but also allows you to contribute to medical progress and improve patient lives worldwide.
Choosing the right career path depends on your interests, skills, and goals. If you’re new to the industry, it’s a good idea to undertake clinical research course along with internship which helps you gain hands-on experience and find out which role suits you best. At ClinverseEdge, as depicted in this figure, we offer internship at premier institutes in India.
Networking with industry professionals and seeking mentorship can also guide you in making the right decisions. Remember, the industry offers many opportunities for growth, and with dedication, you can build a successful career.

You might wonder, “Do I need a medical or pharma degree to succeed?” Not necessarily! Many roles in clinical research are open to students with different backgrounds, as long as you have the right skills and training.
Many organizations now offer online courses and certifications that prepare you for a career in clinical research. These courses cover everything from basic concepts to advanced skills like data analysis, trial management, and regulatory submissions.
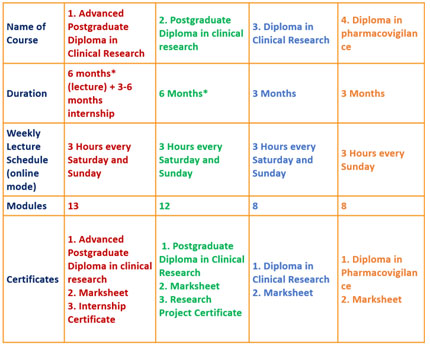
Guided by industry leaders, we also help with placement support, mock interviews, and more to ensure you’re job-ready.

Whether you’re a fresh graduate or a working professional, upskilling online is easier than ever. At ClinverseEdge, we offer expert-led training programs designed to help you succeed, including hands-on experience, real-world scenarios, and exclusive internship opportunities.
Science is your background—now make clinical research your future. Don’t wait for opportunity—create it! Visit https://www.clinverseedge.com/, explore our courses, and start your journey toward a rewarding career in clinical research today.
Remember, the world needs passionate professionals like you to improve healthcare and save lives. Your future in clinical research is just a click away!